Regulatory NewsBREAK: FDA Issues Updated Guidance on Patient-Focused Drug Development
- On November 18, 2025, the Food and Drug Administration (FDA) published a final guidance entitled “Patient-Focused Drug Development: Selecting, Developing, or Modifying Fit-for-Purpose Clinical Outcome Assessments” (Final Guidance).
- This Final Guidance is the third in a series of four guidance documents that describe how stakeholders can submit patient experience and other information for medical product development and regulatory decision-making:
- Guidance 1 covers methods to collect accurate and representative patient experience data for the intended patient population;
- Guidance 2 covers approaches to identifying what is most important to patients about their experience of the burdens of the disease/condition and treatment;
- Guidance 3 (this Final Guidance) covers approaches to selecting, developing, modifying, and evaluating clinical outcome assessments (COAs) to measure outcomes of importance to patients in clinical trials; and
- Guidance 4 covers methods, standards, and technologies for collecting and analyzing COA data for regulatory decision-making, including selecting the COA-based endpoint and determining clinically meaningful treatment effects.
- A COA is a measure that is intended to describe or reflect how a patient feels or functions and can be used to support effectiveness, dose optimization, safety, and tolerability in the context of a clinical trial to determine the clinical benefits and risks of a medical product.
- COAs (including patient-reported outcomes, observer-reported outcomes, clinician-reported outcomes, and performance outcomes), the role of COAs in evaluating clinical benefit for a medical product, and determining if a COA is fit for purpose.
- The Final Guidance also provides a roadmap to patient-focused outcome measurement in clinical trials to help guide the selection, modification, or development of a COA:
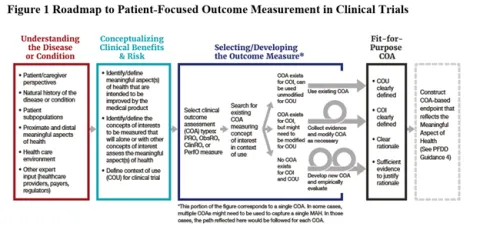
- Finally, the Final Guidance discusses the development of evidence and the components of a well-supported rationale to justify the appropriateness of a COA in a particular context.
For questions, please reach out to Vicky Jucelin.
Featured News & Resources
See Full CalendarUpcoming Events
AMCP offers a wide variety of educational opportunities, from events and webinars to online training.







