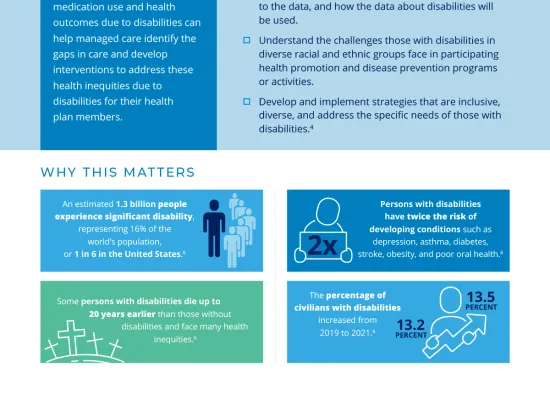
In managed care pharmacy, formulary and utilization management requires collaboration across health care teams (drug review panels, safety committees, medical and pharmacy staff etc.) to ensure the appropriate, safe, and cost-effective use of medications. Our resources provide guidelines, tools, and best practices to help these health care professionals manage drug formularies, optimize medication therapy, and enhance patient care.